In-Depth Content

Technological advancements in the world within the last 50 years have brought society to a place that one could only dream of some years back. The pharmacy field is no stranger to technology and has shown tremendous changes and progress in this area. Only fifteen years ago, many facilities still utilized faxing of paper orders and manual processes during central pharmacy dispensing. IV admixture did not include barcoding; a pull-back method (where a pharmacy technician would compound an admixture and simply fill used syringe barrel with an air, setting the plunger to the mark of supposedly used volume) was a daily practice.
Today, pharmacy operations rely heavily on automated processes. The main reason for this transition is safety, efficiency and reliability. It is a known fact that robotic machinery has a significantly lesser chance of error, as compared to manual fills in medication dispensing. It is also known that barcode scanning while dispensing a medication, or restocking automated a cabinet, significantly reduces chances of mislabeled medications leaving the pharmacy and reaching patients.
When evaluating a new automation system, it is important to consider how it will perform during downtime. Key questions to ask the vendor include:
- Will the equipment use Active Directory login, and if so, will network outages prevent end users from logging in?
- How easily accessible is the medication inventory during downtime?
- Will the equipment remember transactions during downtime, and can these transactions be reconciled later?
- How user-friendly is the system, and can an average technician or pharmacist service it without difficulty?
- How resilient is the equipment to cyber incidents, and will it continue to function during a ransomware attack?
Asking these questions helps build robust processes and ensure system reliability.
The above concept of emergency preparedness was put to the test during the recent CrowdStrike incident. A faulty software update resulted in a devastating downtime event, affecting not only the healthcare industry but also airlines, large and small businesses, and people around the world.
Many hospitals across the country had to delay or cancel their surgical procedures and business operations reverted to paper. This incident exposed the internal vulnerabilities of many automation systems in the pharmacy world, including automated medication dispensing cabinets. The importance of timely, yet controlled software updates come to mind. For the system to operate safely and reliably – scheduled software updates are needed. It is advisable to have those updates manually pushed to medication dispensing cabinets, on schedule, rather than allowed to be automatically loaded. Software feature enhancements should always be carefully reviewed by the designated end-users, prior to load. It is best to have automated updates on medication dispensing cabinets and other vital equipment (such as infusion pumps, robotics, carousels, anesthesia carts) applied manually, on schedule, in collaboration with the facility’s information technology department support.
In today’s pharmacy, automation is used every step of the way from purchasing, to receiving medications, to dispensing, packaging, processing, and administering. Ensuring procedures are in place to enhance patient safety, data integrity, and business continuity is a step that every facility should be working on daily.

In June of 2023, Techdow USA Inc., a division of the Hepalink Group, successfully launched Enoxaparin Sodium (Enoxaparin) in multiple strengths of pre-filled syringes. Techdow USA’s Enoxaparin is critically needed and will be used primarily in the outpatient setting to treat and prevent harmful deep vein thrombosis and pulmonary embolisms. Techdow USA’s parent company, the Hepalink Group, is a global leader in naturally extracted active pharmaceutical ingredients (API) and finished drugs that sources from an extremely diverse and robust supply chain network.
Techdow USA sources materials from North America, Europe, and Asia. Additionally, Hepalink Group owns and operates Scientific Protein Laboratories (SPL), the largest producer of Heparin API in North America. SPL’s API business was created by Oscar Mayer in the 1970s and is a global leader in naturally extracted API production. The Hepalink Group has expanded their worldwide offerings to now include manufacturing and marketing of their high-quality and reliable finished drug products in the USA.
Techdow USA launched Heparin Sodium injection in 2022 followed by their next launch of Enoxaparin Sodium injection. Both Enoxaparin and Heparin Sodium are a complement to the Hepalink Group’s sophisticated vertically integrated products supply chain that provides increased supply reliability, competitive pricing, and robust quality.
The Hepalink Group, a global supplier to the market and trusted partner to the largest pharmaceutical companies, is delighted to provide our customers with the opportunity to directly access the market’s largest global supply of our finished drug products, Enoxaparin and Heparin. Techdow USA’s products provide patients and customers the supply reliability they demand and deserve
Techdow USA is a high-performance pharmaceutical company that is committed to quality and reliability to our patients and customers. We develop, manufacture, and sell critical FDA approved products such as Heparin and Enoxaparin Injection for use in hospital and clinical settings. We are vertically integrated in raw materials and can deliver hard to source products at a competitive price.
Techdow USA is among the fastest growing companies in our industry and are always looking for ways to improve and set a new high-performance standard. We are passionate about making a difference for patients and bringing effective medicines to market.

The healthcare industry is undergoing rapid change, placing pharmacy operations at a critical juncture. As drug prices rise and reimbursement rates fluctuate, health systems are increasingly seeing the untapped potential of retail pharmacy services as a key tool for generating revenue and improving patient care. This shift calls for innovative strategies to maximize revenue in pharmacy settings while maintaining a careful balance between financial sustainability and high-quality patient care.
In recent years, the role of pharmacies within health systems has seen significant change. No longer viewed simply as a cost center, pharmacies are now recognized as potential profit drivers and essential parts of integrated care delivery networks. This shift, highlighted by a recent Premier survey of health system leaders, is not just about financial gains. It reflects a broader approach that addresses financial challenges while also supporting population health efforts and improving patient outcomes.
One of the most promising opportunities for increasing revenue is reducing hospital readmissions. With the annual cost of 30-day readmissions reaching $41.3 billion, or $13,800 per Medicare patient, the financial impact is considerable. Onsite retail pharmacies are in a strong position to address this issue by providing thorough medication counseling before discharge, which significantly reduces the chances of readmissions and emergency department visits.
The advantages of onsite retail pharmacies extend far beyond readmission reduction. Research indicates that nearly 30% of patients fail to fill their initial prescriptions within a week of discharge. The mere presence of an onsite pharmacy serves as a powerful visual catalyst, markedly increasing the probability of prescription fulfillment. It has been proven time and time again across the United States that pharmacists embedded within healthcare systems can offer immediate interventions that their external counterparts often cannot, recommending cost-effective alternatives, facilitating connections with financial assistance programs, and collaborating directly with inpatient care teams to address potentially hazardous prescriptions, thereby enhancing patient safety and care quality.
For eligible hospitals, the 340B Drug Pricing Program represents a significant avenue for revenue enhancement, with participating retail contract pharmacies reporting returns on investment as high as 15%. However, capitalizing on this opportunity demands meticulous record-keeping, regular price file updates, robust tracking systems, and a comprehensive understanding of the regulatory landscape.
Efficient inventory management emerges as another crucial factor in revenue maximization. The implementation of advanced inventory systems, coupled with data analytics for demand prediction and strategic negotiations with wholesalers, can substantially reduce costs and enhance cash flow. Regular evaluations of slow-moving items and consideration of alternative sourcing further optimize inventory operations.
Diversification of revenue streams through expanded clinical services offers yet another avenue for growth. Comprehensive medication therapy management programs, immunization services, and specialty pharmacy offerings for high-cost medications can significantly augment revenue while simultaneously improving patient care.
To truly flourish in this evolving landscape, health systems must think beyond their current operational paradigms. This may involve geographic expansion to high-traffic locations, forging partnerships with local employers to access new patient populations, or implementing mail-order services to enhance convenience, foster customer loyalty, and meeting the consumer where they are at by getting with the times of technology and delivery.
As many organizations continue to scale for success, it is imperative to understand that maximizing revenue capture in pharmacy operations demands a nuanced and multifaceted approach that harmoniously balances strategic expansion, operational efficiency, and an unwavering commitment to patient care. By conceptualizing their retail pharmacies as strategic assets capable of enhancing care delivery, mitigating readmissions, and generating substantial revenue, health systems can position themselves advantageously in the increasingly complex healthcare ecosystem.
As we navigate this transformative era, those who can adeptly maneuver through these intricacies and embrace this comprehensive approach will not only ensure the financial sustainability of their pharmacy operations but will also play a pivotal role in shaping the future trajectory of healthcare delivery. The pharmacy of tomorrow transcends its traditional role as a mere dispensary, emerging as a key player in the dual pursuit of superior health outcomes and financial stability for health systems nationwide.
References: Tarr, L. D., & Lupica, S. (2004). Workers\u27 Compensation. https://core.ac.uk/download/232791407.pdf

We envision a world where women are free to be open and honest about their intimate health, because women care about their health, and they know there is no issue too small to tackle—but they don’t always have the right tools. We believe in providing those tools so women can live life uninhibited—and that means breaking the taboos that surround women’s health. This is not just for AZO® users and not just for women, but also to enrich our society. When everyone is supported, healthy and safe, our world is better.

I strongly believe that every medical center needs a dedicated medication safety leader. The American Society for Health-System Pharmacy (ASHP) published a position statement on the Role of the Medication Safety Leader that I highly encourage all pharmacy directors to read. Medication safety simply cannot be another hat one wears as a pharmacy manager or director who is also managing budgets, monitoring financial performance, implementing cost-saving measures, addressing regulatory concerns, maintaining a competent and engaged staff, and more! A medication safety leader sets the medication safety vision, identifies opportunities to improve the medication-use process, and leads efforts and initiatives to prevent medication errors. For those who have bought into this idea and secured the funding to hire a dedicated medication safety leader, wonderful and congratulations! Here are some tips on what to look for in your future medication safety leader along with how to assess for these traits during the interview. The same ASHP position statement referenced above lists 19 characteristics that medication safety leader should have which I agree with 100%. I am going to add (or highlight) five (5) more.
- Assertive, but not aggressive. “Appropriate assertiveness” is already listed as a desirable characteristic on the position statement (characteristic #15), but I want to call out the need for the appropriate assertiveness and why to avoid aggressiveness. Medication safety leaders are often put into the position of having to convince various levels of leadership of something- to heed the lessons of medication errors, to consider a new process, to implement safety technology, etc. Many of which are unpopular ideas from the start for a variety of reasons: cost, level of effort to implement, increased workload, or even normalcy bias (the concept that an outside error won’t happen here). Appropriate assertiveness refers to advocating for safety to the right stakeholders, at the right forum, and at right time. Being aggressive does not help to convince leadership. Instead, it turns them away. During an interview, ask candidates about how they manage conflict or how they respond to criticism and look for non-verbal cues such as eye-contact and tone to assess for assertiveness.
- Highly organized. At any given moment, medication safety leaders are juggling a multitude of projects and initiatives at various stages – some in flight, some they are still advocating or getting buy-in for, some nearing completion or in the monitoring phase. This is in addition to day-to-day medication error review, presentations, and meetings. It takes a highly organized individual to keep everything straight and moving forward. During the interview, ask candidates how they stay (or plan to stay) organized with multiple projects or ask them to walk you through how they managed a complex project.
- Flexible. In my nine years in medication safety, I have come to realize the need to pivot multiple times with medication safety projects. At times we need to pivot within the project itself i.e. we were headed north, but now realize we need to head northeast (metaphorically speaking). In addition, we often need to pivot our attention, efforts, and energy from one project to another because of organizational priorities or momentum (sometimes when a new error happens it increases interest and speeds up a safety project). During the interview, some of the same questions for assessing the ‘highly organized’ trait may be used to assess the candidate’s level of flexibility. You can also ask how they adjust to changes that they have no control over.
- Collaborative. Nothing in medication safety can be done in a silo. A medication safety leader cannot develop a safer workflow without engaging front line workers and operational leaders. In addition, more often than not, medication safety projects will be multidisciplinary in nature- involving any combination of physicians, nurses, respiratory therapy, central supply, bioengineering, pharmacy, healthcare risk, and others. During the interview, ask candidates to explain how they collaborated with others on a project.
- Curious. Being curious helps to do so many things- develop rapport, learn about a workflow or system, think outside the box, draw connections, and so much more. During the interview, ask candidates to tell you about a time where they had to complete a project but were not given all of the details. Look for how many questions they ask during the interview about the position and the organization as well as the quality of those questions. Routine questions asked by many candidates may not be reflective of natural curiosity, but thoughtful questions that perhaps reference something discussed earlier might point to a naturally curious individual!

PharmEcology® is the exclusive company that can provide your organization with specialized solutions for USP 800 Assessment of Risk requirements and pharmaceutical waste management programs.

It’s two o’clock in the morning when your phone rings. It’s the on-call hospital administrator at your facility. A natural disaster has struck, and your hospital must remain fully operational for the next 72-96 hours with limited external resources. The 96-hour emergency management plan at your hospital is being activated, and a media press conference is set to take place at 5:00 a.m. The next question you hear is, “Do we have enough medications on hand to provide care to our patients for the next 3-4 days?” As the pharmacy director, which medications will you prioritize as the most medically necessary?
While this scenario might seem like something out of a blockbuster movie, you may have already faced it or addressed a similar question during your triennial accreditation survey preparation. At the core of this situation is the crucial question: Is the medication list in your emergency playbook robust enough to meet the challenge, or will it leave you scrambling?
If you haven’t created an essential medications list before, or are looking to revisit your current one, it’s important to start by defining your scope. According to the World Health Organization (WHO), essential medications are those that satisfy the priority health care needs of a population. Your scope consists of two main components: the setting you are covering and the types of medications you are including.
First, determine what your setting needs to plan for. Then, define which medications are essential for your patients. This should reflect the disease prevalence and public health relevance within your patient population, including common conditions, infrequent but possible scenarios, and rare but potential disease states. Both the identification of disease states and the selection of necessary medications should be approached in a multidisciplinary fashion, leveraging the expertise of specialists in each area to pinpoint the truly essential medications.
When creating a disease state-specific medication list, it’s helpful to identify primary and secondary alternatives when possible. For organizations that serve as centralized hubs within a larger health system, it might be most feasible to set a minimum reorder point at 7-8 days for each medication based on system-wide use. This strategy ensures the larger hub can maintain control over product availability during challenging times. However, given the diversity of your patients and the “rare or potential disease states,” this approach can lead to substantial costs and high levels of waste on seldom used but essential products.
For smaller or leaner facilities, establishing reduced reorder points for secondary agents or even a non-stocking status for infrequent secondary agents, when the primary medication is readily available, can help reduce carrying costs. This approach does require closer attention to stock levels to ensure availability and a commitment to daily ordering.
Regardless of the facility size, partnering with vendors to identify replacement product programs and collaborating with other hospitals in the area to understand each location’s stock can help cut costs and reduce waste.
Lastly, establishing a follow-up procedure to ensure the medications on your emergency list remain in stock is essential. If your automation or inventory management system allows you to set standard stock products in both decentralized and centralized inventory, you can align your inventory reports with cycle counts to ensure products are always replaced after expiration and never inadvertently removed.
For hospitals in regions where emergencies are more common, whether year-round or seasonally, consider creating more frequent automated dashboards to alert your buyer of stock issues. Additionally, revisiting your day’s supply based on current patient usage or embedding the emergency medication evaluation within the formulary approval process can help maintain the stability of your list as treatments and needs evolve.
Steps to Consider When Creating an Essential Medication List for Your 96-Hour Emergency Management Plan:
- Form a multidisciplinary team.
- Include representatives from pharmacy, clinical departments, administration, inventory management, and key disaster management members.
- Ensure involvement of specialists for different disease states to get a comprehensive perspective.
- Define essential medications.
- Prioritize medications based on disease prevalence, public health relevance, and patient needs within your specific setting. Identify primary medications and establish secondary alternatives for each condition.
- Validate the list with input from specialists and clinical teams to ensure completeness and accuracy.
- Develop inventory management protocols.
- Set minimum reorder points based on medication use patterns. For centralized hubs, establish a minimum reorder point at 7-8 days for each medication. For smaller or leaner facilities, consider reduced reorder points or non-stocking status for infrequent secondary agents.
- Use automation and inventory management systems to track stock levels and align reports with cycle counts.
- Create automated dashboards to alert buyers of stock issues, especially in regions prone to emergencies.
- Collaborate with vendors and other local hospitals.
- Collaborate with nearby hospitals to create a network of transparency and cost reduction by sharing information on high-cost, low-utilization medications.
- Work with vendors to identify product replacement programs or consignment opportunities to reduce costs and ensure supply.
- Explore options for emergency restocking and rapid replenishment.
- Create a plan for ongoing monitoring and continuous improvement.
- Schedule regular reviews of the essential medication list to update it based on changing patient needs and treatment protocols. Revisit day’s supply based on current patient usage and embed the emergency medication evaluation within the formulary approval process.
- Maintain communication with clinical teams, administration, and other stakeholders to ensure the list remains relevant and effective.

Seven out of 10 pharmacies are understaffed1. When a pharmacy is understaffed, every aspect of the business suffers: employees become overburdened, lines are longer, and customer service deteriorates, resulting in patient and employee churn.
Does this situation sound familiar?
Fortunately, there's a more sustainable solution than the continuous cycle of hiring and training staff – and you’re already familiar with it. Digital transformation, the adoption of online and integrated technology, can bridge the gaps created by staffing shortages to streamline workflows, enhance efficiency, and increase staff capacity.
While the concept sounds nebulous and overwhelming, we’ve identified the most impactful technologies to help you get started on your journey toward modernization.
No. 1: Streamline Transactions with Digital, Self-Service Payments
As a consumer, you use your phone to pay for goods and services nearly daily. From curbside pickup to third-party delivery apps, 82% of Americans use digital payments regularly2.
However, pharmacies and healthcare have been slow to adopt self-service payments due to regulatory concerns. But as dedicated healthcare payment providers have entered the space, it's now an area ripe for transformation and efficiency gains.
When payments are required to be made at the counter, patients arrive surprised by Rx prices, fumbling with payment methods, and asking pharmacists to update billing information, resulting in lengthy and frustrating transactions. At the most critical point in the patient's journey, the pharmacist is spending more time with the patient on operational activity rather than clinical engagement. No wonder pharmacies are the second most common place where Americans spend the most time waiting in lines 3.
Providing digital, self-service patient payments via text or email offers patients convenience while saving pharmacy staff time and effort4. Patients can pay how they want, when they want, and where they want, while pharmacists can focus on the core elements of their job. When patients arrive to pick up their prescriptions, pharmacists can verify their identity, see proof of payment with a QR code from the patient, collect the necessary signatures, and offer counseling with little to no billing support required.
Instead of a 10-minute wait time and an ineffective interaction at the register, the pharmacist and patient benefit from shorter, yet more effective interactions.
No 2.: Eliminate Manual Processes with a Mobile Point of Sale (POS) System
Is your staff wasting time using pen and paper? You're not alone. 71% of providers still use manual processes for collecting payments, signatures, and identification5. This pulls pharmacists and technicians away from their core duties, increases the likelihood of errors, and compromises security.
Manual pen and paper processes are most common during mobile transactions, like meds to beds or curbside pickup, because most point of sale (POS) systems have been reliant on on-premise, hardware-based solutions. But advancements in cloud computing and mobile devices have enabled technology providers to offer mobile, cloud-based systems that work on tablets and laptops without bulky servers.
With a mobile POS that’s integrated with your pharmacy management system (PMS), staff can capture all necessary patient and payment information as the transaction is being conducted6. This enables revenue-driving programs like meds to beds, drive-thru, curbside pickup, and line busting without adding extra workload for your staff. No more frustrating tasks like tracking down signatures with paper, spending hours uploading and scanning, or worrying about accuracy and compliance.
No. 3: Quickly Locate Prescriptions with an Automated Will Call System
In 2021, over 6.5 billion prescriptions were dispensed in the U.S.7 With 66,000 pharmacies in the U.S. that year, we can roughly estimate that each pharmacy handled an average of 98,000 prescriptions annually8.
Think about how long it takes for your staff to find a patient's prescription during pickup. Even if it only takes a minute, that's 98,000 minutes—or 1,633 hours (about 2 months)—wasted each year.
An effective, low-cost way to reduce this time is through an automated will call system. These systems use lights and/or sounds to help pharmacists quickly locate prescription bags. When dispensing a prescription, the pharmacist scans the prescription and bag before hanging it on the rack or in the refrigerator. When the patient arrives, the bag lights up or makes a noise, allowing staff to locate it instantly, reducing wait times from 10-30 minutes down to seconds.
While pharmacy automated will-call systems aren’t necessarily new, a lighted will-call system that integrates into the point of sale (POS) is. This helps streamline vendors and applications leveraged by pharmacy staff for greater efficiency.
Reclaim Time to Achieve More with Less
Imagine a pharmacy where your team can focus on what truly matters while experiencing more manageable workloads. Alleviating staffing shortages and providing better patient and employee experiences starts with technology. By doing the work now to modernize your most time-consuming processes, your staff, patients, and bottom line will benefit for years to come.
Self-service payments, mobile POS systems, and automated will call systems are just the beginning. What innovative solutions will you explore next?
Resources
- Untapped opportunities for health system pharmacies | McKinsey (mckinsey.com)
- New trends in US consumer digital payments |McKinsey (mckinsey.com)
- Consumer Survey: The State of Waiting in Line (2023) | Waitwhile
- Pharmacy Payments Pain Points: How to Avoid Them | Emporos
- 2024 Trends in Healthcare Payments Annual Report | J.P. Morgan (jpmorgan.com)
- mPOS: A Patient-Centered Solution | Emporos
- Total drug prescriptions dispensed U.S. 2009-2022 | Statista (statista.com)
- U.S. National Pharmacy Market Summary | IQVIA (onekeydata.com)

In a previous article, I covered 5 key things that hospital pharmacy leaders need to know about medication safety [SEE: '5 Things You Need to Know NOW About Medication Safety']. Now, we need to put this into action. In this article, I’ll touch on 4 things to jump start medication safety at your organization!
- Review and share reported medication errors. I have a colleague whose career in medication safety started when she was asked to categorize medication errors as part of a modified work assignment (she was a hospital pharmacist back then) while she was transitioning back from maternity leave. She was only asked to categorize the errors but as she read through them, she started asking the question, ‘What are we doing about these errors?’ That was the impetus for the creation of a dedicated medication safety role at her organization. I cannot emphasize enough the power of these reported errors when they are reviewed AND SHARED! Bring awareness to what is happening to both those on the front line as well as leaders above you who can influence and prioritize resources to projects that will reduce the risk of medication errors.
- Utilize the Institute for Safety Medication Practices (ISMP) Newsletters. As a hospital pharmacist many moons ago, I was fortunate enough to be part of an organization that shared the ISMP newsletters with staff. It was eye-opening (and scary!) to read about medication errors that occurred at other organizations. It can also be overwhelming to realize how much risk exists! That said, don’t try to boil the ocean. Instead, identify one or two items each quarter (or even each year) that you want to address for your pharmacy or hospital and just focus on that. While some recommendations may require a heavy lift (e.g., a system enhancement to the organization’s electronic health record), others could be easier to implement and lead to small but quick wins.
- Assign small medication safety related projects to interested staff. As a pharmacy leader, remember that you do not have to do everything. Instead, as medication safety related projects arrise, leverage the talent, expertise, and interest from your staff. This is a win-win-win all the way around. The hospital or pharmacy benefits from the project, the employee is engaged and feels valued for being able to contribute in a unique way, and the manager gets something they has been meaning to address off their plate. Standardizing a process (example: a process for unit dosing medications), creating a quick reference card/table (example: creating a laminated table of where certain medications can be infused based on unit), or redesigning an automated dispensing cabinet matrix drawer or tower to optimize efficiency of refills AND minimize look alike errors, are all very ‘doable’ by staff, with guidance.
- Hire a dedicated medication safety officer! I strongly believe that every medical center needs a dedicated medication safety officer to set the medication safety vision, identify opportunities to improve the medication-use process, and lead efforts and initiatives to prevent medication errors. This simply cannot be another hat that a manager or director wears in addition to a multitude of other responsibilities on their plate. In a later article, I’ll provide insight into what to look for in a medication safety officer.

Children's National Hospital Division of Pharmacy boosted its pharmacy operational efficiency by merging datasets across its workflow platforms into a central data repository, from which many co-variables were then accounted for. With aggregated workload data from 180+ employees across 10,000 shifts, they implemented user-friendly dashboards, ensuring accuracy through collaboration.
The result? Major operational and clinical improvements, highlighting the power of data integration and automation in healthcare. This signals a bright future for efficiency with advancements in automation, predictive AI, and data analytics, poised to revolutionize pharmacy operations and patient care. Upon implementation, one of the Operations Manager voiced being “empowered to track, trend, and share employee metrics during their monthly 1on1s with their direct reports especially now being able to account for differences in workload in different shifts, days, and whether training or not”.
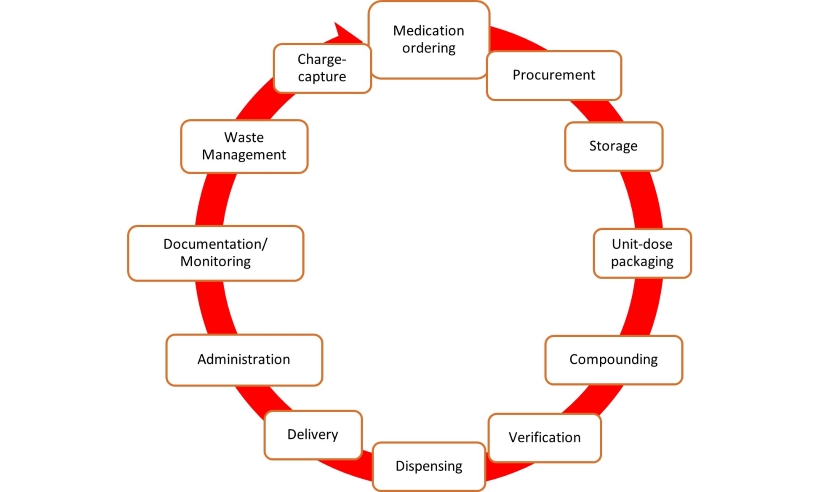
Fig 1: Lifecycle of medication in the hospital. Each stage of the medication lifecycle has different disconnected technology platforms with different datasets needed to be merged into a data warehouse.
Healthcare organizations face a data explosion, with the sector generating a significant share of global data. Despite this, healthcare lags in data utilization due to privacy concerns and disjointed systems. Children’s National Hospital, like many others, operates various disconnected platforms, hindering data analysis and workflow understanding. Manual data handling strains resources, urging the need for automated solutions. To enhance efficiency and patient care, hospitals are turning to digital health strategies, integrating analytics platforms. Children’s National Hospital aimed to streamline pharmacy data management through automation, addressing staffing challenges and burnout. This report outlines the implementation process and key insights gained.
Setting the Stage: Navigating Pharmacy Innovation
Children’s National Hospital, recognized as a top-ranking facility in neonatal intensive care and overall pediatric care by U.S. News & World Report, prioritizes pediatric well-being. With a dedicated team of over 180 professionals, patient safety remains paramount. To improve medication management, the hospital sought modernization. Recognizing the inefficiency of Excel, which consumed 25 hours monthly and incurred extra costs, the pharmacy initiated a digital analytics pilot. Technical hurdles and data inaccuracies necessitated a transition to streamline medication lifecycle management.
The Blueprint for Pharmacy Efficiency
To meet diverse data reporting needs like daily huddle, Department of Health DOH on-demand, and C-suite reporting, an integrated platform was sought. Preceding tool development, similar organizations were studied for best practices. Some of those studied included a rural Maryland hospital analytics-driven approach with reduced spending on acetaminophen by 78%, a multihospital system in Central NC using clinical analytics to monitor high-risk medications, and a large multihospital and clinical system in South Florida streamlining its formulary after acquiring more hospitals and clinics to its network.
Critical platforms were integrated in phase I, with less critical ones and financial data slated for phase II. Real-time reporting measures encompassed dose preparation and verification efficiency, shift efficiency, dispenses to outside clinics, and controlled substance dispenses. Validation involved collaborative efforts between the Division of Pharmacy Services team and the analytics platform provider's Director of Healthcare Consulting and Implementation.
Powering Pharmacy Performance
Post-implementation, the platform analyzed eight real-time datasets, covering over 180 employees and 10,000 shifts, providing comprehensive insights into operational and clinical efficiency. Access to real-time dashboards centralized data from the entire medication lifecycle, allowing for streamlined measurement of performance indicators. Integration of previously siloed platforms enhanced leadership's visibility into employee efficiency, resulting in a 75.5% reduction in open shift hours. The dashboards facilitated accurate staff probation decisions and reduced manual data entry, freeing up 25 hours monthly in bandwidth. Phase II integration included census and capacity data, enabling predictive staffing level assessments with <5% variance to actual.
Navigating Challenges Learned in Pharmacy Automation
The Division of Pharmacy at Children's National learned key lessons in automating and integrating data tracking and would like to share for others considering adopting new data and analytics platforms. They include:
- Buy-in: Securing support from leadership and frontline staff is crucial, emphasizing increased efficiency and improved outcomes.
- Time: Phase 1 took over 300 hours across 6-8 months, requiring adequate bandwidth and avoiding peak busy seasons.
- Technological: Managing IT governance, including HIPAA compliance, and addressing cybersecurity concerns with the IT department are essential. Renegotiating contracts with existing vendors may be necessary.
- Project staff-mix: Some staff lacked a blend of clinical and technical expertise, hindering effective integration and tailoring of the analytics software. Avoid using a non-clinical project manager and rather utilize a pharmacist with both clinical and technical knowledge to facilitate quicker implementation.
The Future of Data Automation in Healthcare
Automating data analysis fits the future of healthcare, aligning with digital health trends. Recent advancements include vaccine management and sterile automated compounding. Predictive analytics and AI are increasingly vital in diagnosis, treatment, and regulation. Predictive AI can enhance pharmacy efficiency by forecasting drug demand and managing medications. Integration with Cerner EHR aims to bolster analytical trends, identify cost-saving opportunities, and improve safety reporting.
Conclusion
Implementing a unified data platform improved pharmacy efficiency and real-time reporting at Children’s National Hospital. Integrating clinical and operational data is crucial for hospital effectiveness. As healthcare automation advances, digital analytics platforms are vital for capturing and enhancing clinical performance.
We urge other healthcare systems to merge data and share results to maximize efficiency and outcomes.