Organizational Procedure

[Original publication date 9/3/2024] - Pharmacy technicians are the backbone of successful pharmacy operations. In health system inpatient pharmacies, they handle complex tasks such as sterile compounding, inventory management, controlled substance security, automation oversight, and handling of hazardous medications. They work tirelessly behind the scenes to ensure patients receive safe and quality pharmaceutical care. The ongoing shortage of qualified pharmacy technicians is a significant barrier to progress in an evolving healthcare landscape in the era of technological revolution.
Over the past decade, almost all states have introduced licensing and national certification requirements for pharmacy technicians. Despite these new requirements, salaries and benefits for these positions have not kept pace, leading to a significant industry lag. The shortage was further exacerbated by increased burnout during the COVID-19 pandemic and a growing workload due to an aging population, stricter regulations around sterile compounding, and increasing complexity of tasks.
In health system inpatient pharmacies, the technician shortage is a major challenge, second only to drug shortages. Recruiting, onboarding, and training technicians are time-consuming processes, and staffing gaps lead to stress, lower morale, and additional vacancies. To address this issue, consider implementing competitive pay and hiring incentives, a career ladder, flexible scheduling, awareness campaigns, and robust training programs.
Competitive Pay and Hiring Incentives: Pharmacy technicians earn between $30,000 and $50,000 annually, with advanced roles reaching $50,000 to $60,000. The Bureau of Labor Statistics projects a 6% increase in job outlook for pharmacy technicians over the next year. Health system pharmacies should partner with local HR teams to conduct a thorough market pay review and implement competitive salaries and benefits to attract candidates. Additional incentives, such as sign-on bonuses and tuition reimbursements, are also effective in drawing more candidates.
Career Ladder: Offering career advancement opportunities can attract high-caliber candidates. These types of candidates are the most productive and committed members at the core of pharmacy operations. Positions like operations coordinator, lead/supervisor, chemotherapy specialist, IT specialist, and quality/regulatory compliance lead are becoming more popular and can attract committed technicians.
Flexible Scheduling: For 24/7 health system inpatient pharmacies, creating schedule flexibility can be challenging. Leaders should review workflow plans to identify opportunities for flexible shift times and adjust schedules to attract candidates who cannot commit to rigid hours. Consider shifting repetitive tasks to more popular hours, such as 8 a.m. to 5 p.m.
Awareness Campaigns: The shortage is partly due to a lack of public awareness about the career prospects for pharmacy technicians. Use social media, local job fairs, and presentations at high schools and colleges to promote the role and attract interest from potential candidates.
Robust Training Programs: Collaborate with local academic institutions offering pharmacy technician programs. These students often struggle to find hospital sites for their externships, so creating opportunities for them can benefit both the candidates and the department to evaluate if it’s a good fit.
In summary, pharmacy technicians are crucial to running safe and efficient pharmacy operations. Addressing the shortage with these strategies can help ensure a steady supply of skilled professionals.

Pharmacy design is evolving, driven by advancements in technology, a deeper understanding of workflow optimization, and an increased focus on medication safety and staff well-being. The future of pharmacy design depends on a comprehensive approach that integrates state-of-the-art technology, ergonomic principles, and a patient-centered mindset. This article explores the trends and key considerations shaping the pharmacy of tomorrow, highlighting how strategic design improvements can enhance efficiency, ensure safety, and improve patient outcomes.
Embracing Technological Advancements
Integration of cutting-edge technology is paramount in modern pharmacy design. As pharmacy operations expand and grow in complexity, leveraging technology is critical to function. Automated dispensing systems, robotic medication management, and advanced inventory tracking should not be futuristic concepts, but indispensable components of an efficient pharmacy. These technologies streamline operations, reduce errors, and allow staff to focus on patient care.
Impact of Technology on Pharmacy Operations
- Reduced Medication Errors: One study reported a 63% decrease in potential adverse drug events and a 96% relative reduction in dispensing errors after implementation of barcode technology in a hospital pharmacy.1
- Increased Efficiency: One hospital pharmacy achieved a 7-minute turnaround time and an average accuracy of 99.48% after implementation of an automated dispensing system.2
- Inventory Optimization: Upgrading dispensing technology and properly managing automated dispensing cabinets can decrease inventory stockouts and generate significant cost savings through enhanced inventory management.3
Prioritizing Ergonomics and Staff Well-Being
The physical design of a pharmacy must prioritize staff well-being, as studies show that employee satisfaction is linked to higher customer satisfaction.4 Ergonomically designed workstations analogous to airplane cockpits reduce physical strain and increase productivity. Poor working conditions have been shown to contribute to medication errors.5 Survey data found that pharmacy personnel who are at risk of experiencing high distress have a twofold risk of making a medication error.6 Intentional staff-centered design can mitigate these risks.
Designing for Medication Safety and Regulatory Compliance
Good workflows significantly impact medication and patient safety, so it should come as no surprise that safe medication practices should be at the forefront of pharmacy design. In a global systematic review, the World Health Organization reported a 5% prevalence of preventable medication-related harm.7 One study at an academic hospital observed that 21% of medication errors went undetected by pharmacists during verification.8 Designing workflows that emphasize safety, combined with a commitment to regulatory compliance, ensures patient protection.
Enhancing Collaboration and Communication
Effective communication and collaboration among pharmacy staff and other healthcare professionals are essential for delivering high-quality care. Convenient access to patient data improves medication management and reduces errors stemming from miscommunication. Pharmacy design should incorporate spaces and workstation lines of sight that facilitate teamwork and information sharing. Quiet workspaces that promote concentration and minimal interruptions are crucial for tasks that require high levels of focus. Distractions are linked to 45% of medication errors.9
Conclusion
Thoughtful pharmacy design has immense potential to transform healthcare and pharmacy operations. By embracing technology, prioritizing ergonomics, ensuring medication safety, and fostering collaboration, we can create efficient pharmacy environments that deliver high-quality patient care, ultimately improving patient outcomes.
References:
- Poon EG, Cina JL, Churchill W, et al. Medication Dispensing Errors and Potential Adverse Drug Events before and after Implementing Bar Code Technology in the Pharmacy. Annals of Internal Medicine. 2006;145(6):426. doi:https://doi.org/10.7326/0003-4819-145-6-200609190-00006
- Temple J, Ludwig B. Implementation and evaluation of carousel dispensing technology in a university medical center pharmacy. American Journal of Health-System Pharmacy. 2010;67(10):821-829. doi:https://doi.org/10.2146/ajhp090307
- Labuhn J, Almeter P, McLaughlin C, Fields P, Turner B. Supply chain optimization at an academic medical center. American Journal of Health-System Pharmacy. 2017;74(15):1184-1190. doi:https://doi.org/10.2146/ajhp160774
- Chamberlain A, Zhao D. The Key to Happy Customers? Happy Employees. Harvard Business Review. Published August 19, 2019. https://hbr.org/2019/08/the-key-to-happy-customers-happy-employees
- Pharmacy Workplace and Well-Being Reporting (PWWR) PWWR Report X Second Quarter 2024. American Pharmacists Association; 2024:1-11. Accessed September 24, 2024. https://www.pharmacist.com/Advocacy/Well-Being-and-Resiliency/pwwr
- Pharmacy Staff | Mental Health Resources | Rising Stress Levels. National Association of Boards of Pharmacy. https://nabp.pharmacy/initiatives/pharmacy-practice-safety/mental-healt…
- World Health Organization. Global Burden of Preventable Medication-Related Harm in Health Care. World Health Organization; 2024.
- Cina JL, Gandhi TK, Churchill W, et al. How Many Hospital Pharmacy Medication Dispensing Errors Go Undetected? The Joint Commission Journal on Quality and Patient Safety. 2006;32(2):73-80. doi:https://doi.org/10.1016/s1553-7250(06)32010-7
- Cohen MR, Smetzer JL. Safe Practice Environment Chapter Proposed by United States Pharmacopeia; Sulfamethoxazole/Trimethoprim and Lisinopril Hyperkalemia. Hospital Pharmacy. 2009;44(3):210-213. doi:https://doi.org/10.1310/hpj4403-210

In hospital settings, medication reconciliation stands as a cornerstone in patient safety efforts, ensuring accurate medication histories and preventing adverse drug events. Pharmacy technicians play a pivotal role in this process, offering indispensable support to pharmacists and healthcare teams. As hospital pharmacy leaders and executives navigate the complexities of healthcare delivery, harnessing the full potential of pharmacy technicians in medication reconciliation becomes paramount for optimizing patient outcomes and operational efficiency.
The Role of Pharmacy Technicians in Medication Reconciliation
Pharmacy technicians are instrumental in ensuring comprehensive and accurate medication reconciliation processes within hospital environments. Their responsibilities include gathering medication histories from patients and caregivers, verifying medication lists, and documenting relevant information for pharmacists and clinicians. By meticulously reviewing discrepancies and collaborating with other healthcare providers, technicians help mitigate risks associated with medication errors and improve overall patient safety.
Moreover, pharmacy technicians are adept at utilizing technology-driven solutions for reconciling medications across transitions of care, such as admissions, transfers, and discharges. Their proficiency in pharmacy information systems and electronic health records enables them to streamline workflows and maintain up-to-date medication records, thereby supporting seamless transitions and continuity of care for patients.
Upson Regional Medical Center (URMC) streamlined their medication reconciliation processes in 2021 when they deployed a single pharmacy technician to the Emergency Department (ED) to assist nursing with obtaining medication histories. This initiative resulted after experiencing increased and continuous reports of medication errors related to medication reconciliation. One-year post implementation, their rate of medication errors related to inappropriate or inaccurate medication histories had reduced by 80%. To this date, URMC has added a second pharmacy technician to the ED, allowing seven-day coverage, and have achieved the Leapfrog standard for medication reconciliation. Heather Riggins, Director of Pharmacy at URMC, states that hiring competent pharmacy technicians and ensuring they are trained up to obtain the best possible medication history is critical.
Strategies for Hiring Qualified Pharmacy Technicians
To bolster medication reconciliation efforts, hospital pharmacy leaders should adopt strategic approaches for hiring qualified pharmacy technicians:
1. Competency-Based Recruitment: Implementing competency-based assessments during the hiring process ensures that candidates possess essential skills in medication reconciliation, attention to detail, and proficiency in pharmaceutical software systems. URMC administers a didactic exam that includes matching generic/trade names for the top 200 drugs, identifying appropriate indications for medications, and hypothetical case studies for critical thinking skills.
2. Collaborative Hiring Practices: Involving pharmacists and competent pharmacy technicians in the recruitment process facilitates the selection of candidates who align with the hospital’s patient safety goals and organizational culture. URMC utilizes peer interviews for all hiring candidates, which includes a tour of the pharmacy and hospital. This gives our entire department buy-in on potential new hires. During the tour, staff can observe how the interviewing candidate presents themselves, interacts with others in the organization, and communicates in a more laid-back setting versus at the head of table. Additionally, the peer interview team conducts a mock patient scenario for medication reconciliation to test the candidate’s attention to detail and communication skills.
3. Continued Professional Development: Emphasizing the importance of ongoing education and certification encourages pharmacy technicians to stay abreast of evolving practices in medication reconciliation and enhances their contribution to patient care. URMC candidates are encouraged to obtain specialized training and receive reimbursement for these efforts.
Developing a Strong Training Program
Creating a structured training program is integral to preparing pharmacy technicians for their role in medication reconciliation:
1. Comprehensive Orientation: Offering thorough orientation sessions familiarizes new hires with hospital protocols, medication reconciliation procedures, and the importance of patient safety standards. Society of Hospital Medicine Train the Trainer Materials provides an excellent supplemental guide for training.
2. Hands-On Experience: Providing opportunities for practical training under the supervision of experienced pharmacists and pharmacy technicians allows new hires to refine their skills in medication history taking, data entry accuracy, and effective communication with patients and healthcare teams.
3. Continuous Feedback and Evaluation: Implementing regular performance evaluations and feedback sessions enables technicians to identify areas for improvement and ensures adherence to quality standards in medication reconciliation practices.
4. Integration of Technology: Incorporating training modules on pharmacy information systems and electronic health records equips technicians with the necessary tools to facilitate efficient medication reconciliation processes.
By investing in the recruitment of skilled pharmacy technicians and cultivating a culture of continuous learning and development, hospital pharmacy leaders can strengthen medication reconciliation initiatives. Empowering pharmacy technicians with the knowledge, resources, and support they need fosters collaborative healthcare environments where patient safety remains the top priority.
The role of pharmacy technicians in medication reconciliation is indispensable for ensuring accurate medication histories and promoting patient safety in hospital settings. Through strategic recruitment practices and comprehensive training programs, pharmacy leaders can harness the full potential of technicians to optimize medication reconciliation processes and enhance overall healthcare outcomes. By prioritizing these efforts, hospitals can uphold the highest standards of care and improve the well-being of patients across transitions of care.
References:
https://www.hospitalmedicine.org/clinical-topics/medication-reconciliat…

Technological advancements in the world within the last 50 years have brought society to a place that one could only dream of some years back. The pharmacy field is no stranger to technology and has shown tremendous changes and progress in this area. Only fifteen years ago, many facilities still utilized faxing of paper orders and manual processes during central pharmacy dispensing. IV admixture did not include barcoding; a pull-back method (where a pharmacy technician would compound an admixture and simply fill used syringe barrel with an air, setting the plunger to the mark of supposedly used volume) was a daily practice.
Today, pharmacy operations rely heavily on automated processes. The main reason for this transition is safety, efficiency and reliability. It is a known fact that robotic machinery has a significantly lesser chance of error, as compared to manual fills in medication dispensing. It is also known that barcode scanning while dispensing a medication, or restocking automated a cabinet, significantly reduces chances of mislabeled medications leaving the pharmacy and reaching patients.
When evaluating a new automation system, it is important to consider how it will perform during downtime. Key questions to ask the vendor include:
- Will the equipment use Active Directory login, and if so, will network outages prevent end users from logging in?
- How easily accessible is the medication inventory during downtime?
- Will the equipment remember transactions during downtime, and can these transactions be reconciled later?
- How user-friendly is the system, and can an average technician or pharmacist service it without difficulty?
- How resilient is the equipment to cyber incidents, and will it continue to function during a ransomware attack?
Asking these questions helps build robust processes and ensure system reliability.
The above concept of emergency preparedness was put to the test during the recent CrowdStrike incident. A faulty software update resulted in a devastating downtime event, affecting not only the healthcare industry but also airlines, large and small businesses, and people around the world.
Many hospitals across the country had to delay or cancel their surgical procedures and business operations reverted to paper. This incident exposed the internal vulnerabilities of many automation systems in the pharmacy world, including automated medication dispensing cabinets. The importance of timely, yet controlled software updates come to mind. For the system to operate safely and reliably – scheduled software updates are needed. It is advisable to have those updates manually pushed to medication dispensing cabinets, on schedule, rather than allowed to be automatically loaded. Software feature enhancements should always be carefully reviewed by the designated end-users, prior to load. It is best to have automated updates on medication dispensing cabinets and other vital equipment (such as infusion pumps, robotics, carousels, anesthesia carts) applied manually, on schedule, in collaboration with the facility’s information technology department support.
In today’s pharmacy, automation is used every step of the way from purchasing, to receiving medications, to dispensing, packaging, processing, and administering. Ensuring procedures are in place to enhance patient safety, data integrity, and business continuity is a step that every facility should be working on daily.

It’s two o’clock in the morning when your phone rings. It’s the on-call hospital administrator at your facility. A natural disaster has struck, and your hospital must remain fully operational for the next 72-96 hours with limited external resources. The 96-hour emergency management plan at your hospital is being activated, and a media press conference is set to take place at 5:00 a.m. The next question you hear is, “Do we have enough medications on hand to provide care to our patients for the next 3-4 days?” As the pharmacy director, which medications will you prioritize as the most medically necessary?
While this scenario might seem like something out of a blockbuster movie, you may have already faced it or addressed a similar question during your triennial accreditation survey preparation. At the core of this situation is the crucial question: Is the medication list in your emergency playbook robust enough to meet the challenge, or will it leave you scrambling?
If you haven’t created an essential medications list before, or are looking to revisit your current one, it’s important to start by defining your scope. According to the World Health Organization (WHO), essential medications are those that satisfy the priority health care needs of a population. Your scope consists of two main components: the setting you are covering and the types of medications you are including.
First, determine what your setting needs to plan for. Then, define which medications are essential for your patients. This should reflect the disease prevalence and public health relevance within your patient population, including common conditions, infrequent but possible scenarios, and rare but potential disease states. Both the identification of disease states and the selection of necessary medications should be approached in a multidisciplinary fashion, leveraging the expertise of specialists in each area to pinpoint the truly essential medications.
When creating a disease state-specific medication list, it’s helpful to identify primary and secondary alternatives when possible. For organizations that serve as centralized hubs within a larger health system, it might be most feasible to set a minimum reorder point at 7-8 days for each medication based on system-wide use. This strategy ensures the larger hub can maintain control over product availability during challenging times. However, given the diversity of your patients and the “rare or potential disease states,” this approach can lead to substantial costs and high levels of waste on seldom used but essential products.
For smaller or leaner facilities, establishing reduced reorder points for secondary agents or even a non-stocking status for infrequent secondary agents, when the primary medication is readily available, can help reduce carrying costs. This approach does require closer attention to stock levels to ensure availability and a commitment to daily ordering.
Regardless of the facility size, partnering with vendors to identify replacement product programs and collaborating with other hospitals in the area to understand each location’s stock can help cut costs and reduce waste.
Lastly, establishing a follow-up procedure to ensure the medications on your emergency list remain in stock is essential. If your automation or inventory management system allows you to set standard stock products in both decentralized and centralized inventory, you can align your inventory reports with cycle counts to ensure products are always replaced after expiration and never inadvertently removed.
For hospitals in regions where emergencies are more common, whether year-round or seasonally, consider creating more frequent automated dashboards to alert your buyer of stock issues. Additionally, revisiting your day’s supply based on current patient usage or embedding the emergency medication evaluation within the formulary approval process can help maintain the stability of your list as treatments and needs evolve.
Steps to Consider When Creating an Essential Medication List for Your 96-Hour Emergency Management Plan:
- Form a multidisciplinary team.
- Include representatives from pharmacy, clinical departments, administration, inventory management, and key disaster management members.
- Ensure involvement of specialists for different disease states to get a comprehensive perspective.
- Define essential medications.
- Prioritize medications based on disease prevalence, public health relevance, and patient needs within your specific setting. Identify primary medications and establish secondary alternatives for each condition.
- Validate the list with input from specialists and clinical teams to ensure completeness and accuracy.
- Develop inventory management protocols.
- Set minimum reorder points based on medication use patterns. For centralized hubs, establish a minimum reorder point at 7-8 days for each medication. For smaller or leaner facilities, consider reduced reorder points or non-stocking status for infrequent secondary agents.
- Use automation and inventory management systems to track stock levels and align reports with cycle counts.
- Create automated dashboards to alert buyers of stock issues, especially in regions prone to emergencies.
- Collaborate with vendors and other local hospitals.
- Collaborate with nearby hospitals to create a network of transparency and cost reduction by sharing information on high-cost, low-utilization medications.
- Work with vendors to identify product replacement programs or consignment opportunities to reduce costs and ensure supply.
- Explore options for emergency restocking and rapid replenishment.
- Create a plan for ongoing monitoring and continuous improvement.
- Schedule regular reviews of the essential medication list to update it based on changing patient needs and treatment protocols. Revisit day’s supply based on current patient usage and embed the emergency medication evaluation within the formulary approval process.
- Maintain communication with clinical teams, administration, and other stakeholders to ensure the list remains relevant and effective.

In a previous article, I covered 5 key things that hospital pharmacy leaders need to know about medication safety [SEE: '5 Things You Need to Know NOW About Medication Safety']. Now, we need to put this into action. In this article, I’ll touch on 4 things to jump start medication safety at your organization!
- Review and share reported medication errors. I have a colleague whose career in medication safety started when she was asked to categorize medication errors as part of a modified work assignment (she was a hospital pharmacist back then) while she was transitioning back from maternity leave. She was only asked to categorize the errors but as she read through them, she started asking the question, ‘What are we doing about these errors?’ That was the impetus for the creation of a dedicated medication safety role at her organization. I cannot emphasize enough the power of these reported errors when they are reviewed AND SHARED! Bring awareness to what is happening to both those on the front line as well as leaders above you who can influence and prioritize resources to projects that will reduce the risk of medication errors.
- Utilize the Institute for Safety Medication Practices (ISMP) Newsletters. As a hospital pharmacist many moons ago, I was fortunate enough to be part of an organization that shared the ISMP newsletters with staff. It was eye-opening (and scary!) to read about medication errors that occurred at other organizations. It can also be overwhelming to realize how much risk exists! That said, don’t try to boil the ocean. Instead, identify one or two items each quarter (or even each year) that you want to address for your pharmacy or hospital and just focus on that. While some recommendations may require a heavy lift (e.g., a system enhancement to the organization’s electronic health record), others could be easier to implement and lead to small but quick wins.
- Assign small medication safety related projects to interested staff. As a pharmacy leader, remember that you do not have to do everything. Instead, as medication safety related projects arrise, leverage the talent, expertise, and interest from your staff. This is a win-win-win all the way around. The hospital or pharmacy benefits from the project, the employee is engaged and feels valued for being able to contribute in a unique way, and the manager gets something they has been meaning to address off their plate. Standardizing a process (example: a process for unit dosing medications), creating a quick reference card/table (example: creating a laminated table of where certain medications can be infused based on unit), or redesigning an automated dispensing cabinet matrix drawer or tower to optimize efficiency of refills AND minimize look alike errors, are all very ‘doable’ by staff, with guidance.
- Hire a dedicated medication safety officer! I strongly believe that every medical center needs a dedicated medication safety officer to set the medication safety vision, identify opportunities to improve the medication-use process, and lead efforts and initiatives to prevent medication errors. This simply cannot be another hat that a manager or director wears in addition to a multitude of other responsibilities on their plate. In a later article, I’ll provide insight into what to look for in a medication safety officer.

Children's National Hospital Division of Pharmacy boosted its pharmacy operational efficiency by merging datasets across its workflow platforms into a central data repository, from which many co-variables were then accounted for. With aggregated workload data from 180+ employees across 10,000 shifts, they implemented user-friendly dashboards, ensuring accuracy through collaboration.
The result? Major operational and clinical improvements, highlighting the power of data integration and automation in healthcare. This signals a bright future for efficiency with advancements in automation, predictive AI, and data analytics, poised to revolutionize pharmacy operations and patient care. Upon implementation, one of the Operations Manager voiced being “empowered to track, trend, and share employee metrics during their monthly 1on1s with their direct reports especially now being able to account for differences in workload in different shifts, days, and whether training or not”.
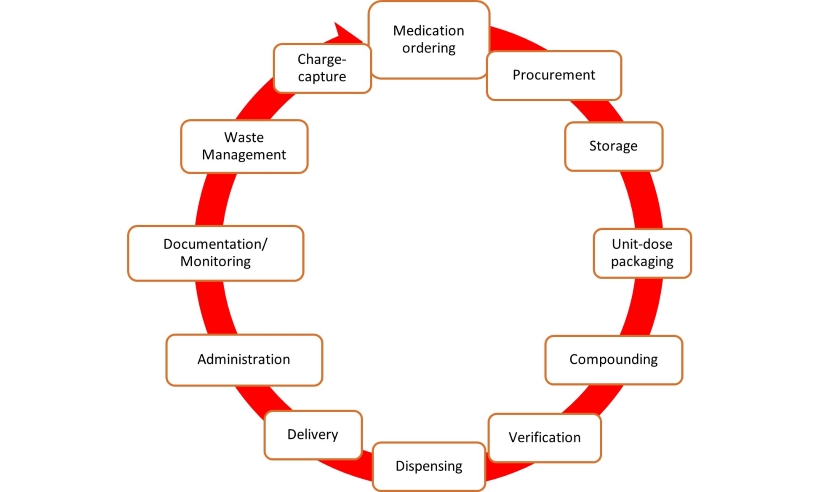
Fig 1: Lifecycle of medication in the hospital. Each stage of the medication lifecycle has different disconnected technology platforms with different datasets needed to be merged into a data warehouse.
Healthcare organizations face a data explosion, with the sector generating a significant share of global data. Despite this, healthcare lags in data utilization due to privacy concerns and disjointed systems. Children’s National Hospital, like many others, operates various disconnected platforms, hindering data analysis and workflow understanding. Manual data handling strains resources, urging the need for automated solutions. To enhance efficiency and patient care, hospitals are turning to digital health strategies, integrating analytics platforms. Children’s National Hospital aimed to streamline pharmacy data management through automation, addressing staffing challenges and burnout. This report outlines the implementation process and key insights gained.
Setting the Stage: Navigating Pharmacy Innovation
Children’s National Hospital, recognized as a top-ranking facility in neonatal intensive care and overall pediatric care by U.S. News & World Report, prioritizes pediatric well-being. With a dedicated team of over 180 professionals, patient safety remains paramount. To improve medication management, the hospital sought modernization. Recognizing the inefficiency of Excel, which consumed 25 hours monthly and incurred extra costs, the pharmacy initiated a digital analytics pilot. Technical hurdles and data inaccuracies necessitated a transition to streamline medication lifecycle management.
The Blueprint for Pharmacy Efficiency
To meet diverse data reporting needs like daily huddle, Department of Health DOH on-demand, and C-suite reporting, an integrated platform was sought. Preceding tool development, similar organizations were studied for best practices. Some of those studied included a rural Maryland hospital analytics-driven approach with reduced spending on acetaminophen by 78%, a multihospital system in Central NC using clinical analytics to monitor high-risk medications, and a large multihospital and clinical system in South Florida streamlining its formulary after acquiring more hospitals and clinics to its network.
Critical platforms were integrated in phase I, with less critical ones and financial data slated for phase II. Real-time reporting measures encompassed dose preparation and verification efficiency, shift efficiency, dispenses to outside clinics, and controlled substance dispenses. Validation involved collaborative efforts between the Division of Pharmacy Services team and the analytics platform provider's Director of Healthcare Consulting and Implementation.
Powering Pharmacy Performance
Post-implementation, the platform analyzed eight real-time datasets, covering over 180 employees and 10,000 shifts, providing comprehensive insights into operational and clinical efficiency. Access to real-time dashboards centralized data from the entire medication lifecycle, allowing for streamlined measurement of performance indicators. Integration of previously siloed platforms enhanced leadership's visibility into employee efficiency, resulting in a 75.5% reduction in open shift hours. The dashboards facilitated accurate staff probation decisions and reduced manual data entry, freeing up 25 hours monthly in bandwidth. Phase II integration included census and capacity data, enabling predictive staffing level assessments with <5% variance to actual.
Navigating Challenges Learned in Pharmacy Automation
The Division of Pharmacy at Children's National learned key lessons in automating and integrating data tracking and would like to share for others considering adopting new data and analytics platforms. They include:
- Buy-in: Securing support from leadership and frontline staff is crucial, emphasizing increased efficiency and improved outcomes.
- Time: Phase 1 took over 300 hours across 6-8 months, requiring adequate bandwidth and avoiding peak busy seasons.
- Technological: Managing IT governance, including HIPAA compliance, and addressing cybersecurity concerns with the IT department are essential. Renegotiating contracts with existing vendors may be necessary.
- Project staff-mix: Some staff lacked a blend of clinical and technical expertise, hindering effective integration and tailoring of the analytics software. Avoid using a non-clinical project manager and rather utilize a pharmacist with both clinical and technical knowledge to facilitate quicker implementation.
The Future of Data Automation in Healthcare
Automating data analysis fits the future of healthcare, aligning with digital health trends. Recent advancements include vaccine management and sterile automated compounding. Predictive analytics and AI are increasingly vital in diagnosis, treatment, and regulation. Predictive AI can enhance pharmacy efficiency by forecasting drug demand and managing medications. Integration with Cerner EHR aims to bolster analytical trends, identify cost-saving opportunities, and improve safety reporting.
Conclusion
Implementing a unified data platform improved pharmacy efficiency and real-time reporting at Children’s National Hospital. Integrating clinical and operational data is crucial for hospital effectiveness. As healthcare automation advances, digital analytics platforms are vital for capturing and enhancing clinical performance.
We urge other healthcare systems to merge data and share results to maximize efficiency and outcomes.

The issue of drug shortages is an enduring problem, dating back to the early 1920s with the first traceable shortage being insulin. National tracking of drug shortages began in 2001, highlighting the persistent nature of this challenge. Despite over a century worth of awareness and opportunity, patients and healthcare providers continue to grapple with drug shortages. Although the idea of “drug shortages” is not exclusive to certain regions or facility type, each healthcare entity navigates these overlapping challenges in its own unique way, reflecting the widespread but varied impact of this ongoing issue.
We started the first quarter of 2024 by breaking historical records, reaching an all-time high of 323 active drug shortages, with nearly half being generic sterile injectable (GSI) medications. The challenges in patient care due to interruptions in GSI availability, coupled with financial implications, are significant. It's estimated that hospitals collectively spend upwards of $600 million per year managing drug shortages. This includes direct costs associated with more expensive, non-preferred products, increased labor costs due to alternative preparation requirements, higher operational carrying costs from increased standard stock amounts, and perhaps most frustratingly, waste once the shortage is resolved.
While no product interruption is ideal, some are more understandable than others. These include manufacturing delays due to equipment maintenance or repair, precautionary batch failures due to suspected quality control issues, or natural disasters. On the flip side, shortages caused by single-source producers dominating the market, significant violations leading to an FDA Form 483, or a diluted product margin resulting in negative value-added situations across multiple manufacturers are less palatable within a fragile system responsible for the health of a nation.
More recently, in response to these concerns, the U.S. Department of Health and Human Services (HHS) released a jointly sponsored white paper titled "Policy Considerations to Prevent Drug Shortages and Mitigate Supply Chain Vulnerabilities in the United States." In this white paper, HHS emphasized the importance of developing and implementing a collaborative, shared accountability model for drug shortage prevention. This model consists of two key components: the Manufacturer Resiliency Assessment Program (MRAP) and the Hospital Resilient Supply Program (HRSP).
The combined program aims to enhance market transparency for GSIs, promote volume-based purchase decisions with a sustainability focus rather than a "race-to-the-bottom" approach, and improve reliability by incentivizing investments in supply chain resilience and diversification. This includes initiatives such as expanding domestic manufacturing and production of key ingredients. However, there's also the potential for hospitals to face penalties under this framework.
Although an official statute has not yet been enacted into law, this is a critical issue. Institutions should take this opportunity to thoughtfully develop and refine clear processes and policies for managing drug shortages at the local level. The following factors should be considered when constructing your inventory management plans during a shortage and preparing to understand your financial position throughout.
Important discussion points to consider when developing your drug shortage management process:
- Work closely with your external pharmacy procurement stakeholders. Vendors such as drug manufacturers, wholesalers, and Group Purchasing Organizations (GPOs) can be invaluable partners. Each partner has the potential to add significant value to your organization.
- Internally, build relationships with key stakeholders across all care areas that may be impacted by a drug shortage. This ensures you understand who can be involved in developing a detailed mitigation plan.
- Determine the appropriate level of inventory for your facility and establish protocols for adjusting standard stock amounts during a shortage. This is a crucial component of financial stewardship during a shortage. Avoid panic-buying all available products in every dose and size to prevent product wastage and tighter constraints caused by a bullwhip effect.
- Conduct frequent huddles with purchasing team members to maintain a consistent understanding of product availability in the market. Daily structured huddles, even if brief, are ideal. Provide broader communication on a weekly basis to all pharmacy leadership for situational awareness, with meaningful escalation as needed. For larger Integrated Delivery Networks (IDNs), establish a system-level committee to review new, active, and pending mitigation plans.
- Organize a response process for activating a drug shortage plan and proactively plan mitigation strategies when monitoring indicates a potential shortage. Start discussions early with key pharmacy staff and select providers to determine preferred treatment alternatives. Do not assume that a pending shortage will be automatically communicated to the relevant stakeholders.
- Leverage your informatics and automation technologies to alert and guide providers to the shortage mitigation strategy, ensuring that any available product is reserved for patients who need it most.
- Identify ways to stretch your current supply to benefit as many patients as possible. Strategies include reducing automated dispensing cabinet PARs, strategically planning compounding for products that contain more than one dose, and shifting inventory from low-use to high-use areas. Work with key internal stakeholders to ensure changes are made with full awareness, for instance in the previous examples, ensure billing units do not result in overcharging during batch preparation of doses or that a PAR reduction does not interfere with urgent response protocols.
- Finally, revisit partnerships and how they can support you during drug shortages. Partners such as wholesalers, GPOs, and manufacturers can collaborate on plans to purchase short-dated products that would otherwise be wasted or enroll in commitment programs that ensure sustainable product availability through predictable and consistent volume needs. Both approaches can reduce your financial burden during shortages, with commitment programs offering proactive shortage prevention; understanding that these programs are most impactful prior to a shortage occurring.

Soaring drug prices are putting the squeeze on pharmacies, but you have the power to resist the trend. Advanced analytics can help your pharmacy loosen hyperinflation’s grip.
From 2022 to 2023, drug prices surged by 15.2%, on average, on top of an 11.2% increase the previous year, significantly outpacing inflation. This past year’s increase constitutes an average difference of $590 per drug, putting big pressure on providers nationwide.
The increases are especially bad news for rural hospitals, where over 600 facilities, or 3 in 10, were at risk of closure in 2023 due to financial instability.
Mitigating the impact of rising costs is an industry priority: 71% of hospital pharmacy participants recently stated that streamlining pharmacy purchasing and procurement was an essential priority for them this year.
The good news is they can do just that.
Where’s the opportunity?
There’s plenty of opportunity among the 40,000 to 60,000 NDC prices hospitals must evaluate daily, given nearly 2,500 preferred NDCs and eight NDC options per therapeutic group.
Most hospitals have been managing this supply chain with low-tech tools like Excel. Many also rely on traditional siloed GPO tools, wholesale portals, and TPA software. Despite pharmacies’ best efforts, this fragmentation hampers data-driven decision-making, creating gaps, deficiencies, and waste.
Traditional procurement approaches also prioritize compliance over financial performance, missing potential improvements in fiscal optimization and sustainability. Even hospitals using newer analytics focus mostly on inventory tracking and supplier consolidation.
Cost-reduction reality
Pharmacy supply chain teams need more. In my view, they need integrated analytics created specifically to optimize spending through blended prices, real-time cost pricing data, and clear visibility into potential savings.
Hospital executives generally understand this need and intend to address it. More than half say data-driven improvements in supply chain management can improve margins by at least 1% to 3%, according to a Sage Growth Partners Survey. The challenge, however, has been creating the right solution.
Fortunately, some hospital systems are adopting purpose-built innovations that surface potential cost savings and elevate the highest-potential opportunities to the top of an integrated dashboard.
These solutions provide transparency around all available drug options to ensure a hospital system gets the best prices and is fully stocked with the drugs it needs. From 2023 to 2024, there was a significant increase in the adoption of new software technologies to help combat purchasing problems, with 25% of respondents turning away from their wholesaler tools compared to 8% the previous year.
Millions of dollars recouped
We looked at 140 hospitals using state-of-the-art purchasing-optimization technology and workflows developed by pharmacists. These leaders saved $27 million in 2023 through real-time data-driven recommendations for better purchasing options. The recommendations considered preferred NDCs, GPO compliance, availability, and WAC, GPO, and 340B ratios.
For the pharmacy professional, a useful real-time purchasing-optimization dashboard provides three simple workflows:
- A direct savings workflow that flags overcharges and recommends lower-priced options for identical products and hard-dollar ROI.
- A cost avoidance workflow that flags 340B insights, contract management opportunities, and impending price increases, preventing overcharges before they happen.
- An oversight workflow that monitors spending, shortages, and GPO compliance.
Relief is in reach
Now, any hospital system can have a bird’s-eye perspective of these workflows in their organization, viewing similar sites in “groups” and facilitating standardized buying practices where applicable. Buyers can dive deeper into any recommended change with a simple click.
Developing such a system doesn’t have to be a major IT undertaking. It’s more about gathering primary data: a wholesaler account list, a 12-month inventory history, 340B covered entity status, and GPO’s preferred NDC list. Standard templates help pharmacy departments connect to major wholesalers.
As a hospital pharmacy, there’s not a lot you can do to prevent drug prices from rising. But you can do more to find the best available price for every purchase. Analytics, dashboards, and the data you already have point the way.

In an evolving time for Pharmacies across the nation, picture a blank canvas. You may ask why? What does this have to do with pharmacy and healthcare? I want you to take a journey with me to the small beginnings I did not despise when it came to our Pharmacy department.
Insight Hospital Chicago is the oldest hospital in Chicago. Its located on the south side of Chicago in a community where the age expectancies vary between 10 minutes of each other. The hospital is a safety net and prizes on the 340B drug program. The spring of 2022 presented the real test. Insight was 100% independent from the previous company. The pharmacy department was a blank canvas with great potential for success.
One of the first discussions was to become dually accredited as a specialty pharmacy. My physical perception of that goal was questionable. We were only an outpatient retail pharmacy servicing a lower number of the hospital and community every week. My inward perception was based on faith that all things are possible. This was the passion that drove us to achieve this vision. When it came to specialty accreditation, we decided to pursue Utilization Review Accreditation Commission (URAC) first. The blank canvas was beginning to have color now. URAC accreditation was very extensive and intense. We rebuilt and reshaped the outpatient pharmacy in many ways. The policies, procedures and workflow were targets that help to provide infrastructure to the department. Within less than one year the outpatient received approval for full accreditation with no findings. This was a mark in history for the pharmacy department and for the hospital. Who would have thought the pharmacy would be able to achieve this in such a quick time. The doors were opening now in many ways. We were now able to get access to limited distributions drugs (LDD), had the payors open more to the pharmacy which helped to support the business. The hospital also gained more awareness in the community. The canvas was becoming fuller and more colorful.
The next accreditation we received for 9 months was Accreditation Commission for Health Care (ACHC). After this second accreditation, the outpatient pharmacy was put on the map. To become dual accredited within one year served as a success and legacy. Our Insight Chicago Pharmacy has gained more traction and notoriety. The canvas went from being blank and empty to full, vibrant and history in the making. Our pharmacy department considered the small beginnings and used that as a guide for the legacy we have now.
Telehealth is a program that has taking off with the Pharmacy at Insight. Through the Collaborative Drug Therapy Management (CDTM) program, the team is able to partner with providers and give the best patient experience. With this program in place, our prescription volume has exponentially increased from where we started. We can now firmly say the pharmacy department at Insight Chicago is flourishing. This also provides great support for the hospital, which is a big pillar in the community. The canvas is now filled and beautiful. It is diverse and versatile. It wouldn’t have gotten this way without a team. Everyone in the pharmacy department worked as a team. From the pharmacy technician to the Chief Pharmacy Officer (COP), there was communication and teamwork. This is what made the vision manifest and come to life!
To conclude, all things are possible when it comes to new beginnings. Health systems across the nation have pharmacy departments that have been long standing and already build our full workflow and operations. The question becomes, what happens when you meet a pharmacy department that is not? Where do you start on making it fully functional, unique and with purpose? Consider the blank canvas journey that I went through and know there are open skies of opportunities. One can start with an vision and believing from within.